Answer: The molarity of this solution is 0.159 M.
Step-by-step explanation:
Given: Mass of solute = 16.3 g
Volume = 1.75 L
Number of moles is defined as the mass of substance divided by its molar mass.
Hence, moles of NaCl (molar mass = 58.44 g/mol) ar calculated as follows.
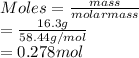
Molarity is the number of moles of a substance present in a liter of solution.
So, molarity of the given solution is calculated as follows.
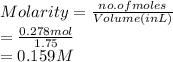
Thus, we can conclude that the molarity of this solution is 0.159 M.