Answer:
Q = 797.5 cal
Step-by-step explanation:
Given that,
Mass of iron, m = 50 g
The temperature rises from 55°C to 200°C.
We need to find the heat needed to raise the temperature. The heat raised is given by :
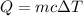
Put all the values,
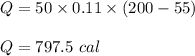
So, 797.5 calories of heat is needed.