Answer:
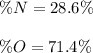
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible to calculate the percent compositions, by dividing the mass of each element by the sum of the masses of the elements as shown below:
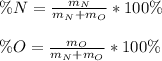
Thus, we plug in the given masses to obtain:
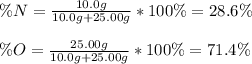
Regards!