Answer:

Step-by-step explanation:
We want to find the new volume given the temperature, so we use Charles's Law. This states the volume of a gas is directly proportional to the temperature. The formula is:
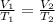
We know the original volume is 20.0 liters and the temperature is 23.0 degrees Celsius. The temperature changes to 45.0 degrees Celsius, but we don't know the new volume. Substitute the known values into the formula.
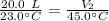
We are solving the new volume, so we need to isolate the variable V₂. It is being divided by 45.0 degrees Celsius. The inverse of division is multiplication, so we multiply both sides by 45.0 °C.
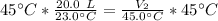
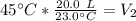
The units of degrees Celsius cancel.
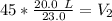
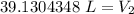
If we round to the nearest hundredth, the 0 in the thousandth place tells us to leave the 3.
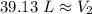
The new volume of the balloon is approximately 39.13 Liters.