Answer:
To make a 4.00 M solution, 48 moles of solute will be needed if 12.0 liters of solution are required.
Step-by-step explanation:
Molar concentration or molarity is a measure of the concentration of a solute in a solution, be it some molecular, ionic or atomic species.
Molarity is defined as the number of moles of solute that are dissolved in a given volume. Then it is calculated as the quotient between the number of moles of solute and the volume of solution:
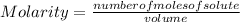
Molarity is expressed in units
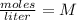 .
.
In this case:
- Molarity= 4 M
- numbre of moles of solute= ?
- volume= 12 liters
Replacing:
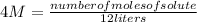
Solving:
4 M*12 liters= number of moles of solute
48 moles= number of moles of solute
To make a 4.00 M solution, 48 moles of solute will be needed if 12.0 liters of solution are required.