Answer:
Its pressure will be 0.54 atm at 100 K.
Step-by-step explanation:
Gay-Lussac's law indicates that, as long as the volume of the container containing the gas is constant, as the temperature increases, the gas molecules move faster. Then the number of collisions with the walls increases, that is, the pressure increases. That is, the pressure of the gas is directly proportional to its temperature.
Gay-Lussac's law can be expressed mathematically as the quotient between pressure and temperature equal to a constant:
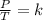
Studying two different states, an initial state 1 and a final state 2, it is satisfied:
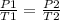
In this case:
- P1= 1.75 atm
- T1= 50 °C= 323 K (being 0 C=273 K)
- P2= ?
- T2= 100 K
Replacing:
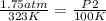
Solving:
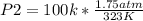
P2= 0.54 atm
Its pressure will be 0.54 atm at 100 K.