Answer:

Step-by-step explanation:
This question asks us to find the new volume if the pressure changes. Therefore, we use Boyle's Law, which states that pressure and the volume of the gas are inversely proportional. The formula is:
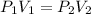
We know that the original sample of gas occupies 4.5 liters at a pressure of 1.63 atmospheres. We know that the pressure was changed to 2.4 atmospheres, but we don't know the volume. Substitute all known values into the formula.
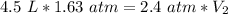
Since we are solving for the new volume (V₂), we need to isolate the variable. It is being multiplied by 2.4 atmospheres and the inverse of multiplication is division. Divide both sides by 2.4 atm.
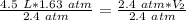
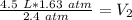
The units of atmospheres (atm) cancel.
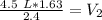
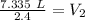
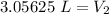
The smallest number of significant figures in the original measurements is 2, so our answer must have the same. For the number we found, that is the tenths place.
The 5 in the hundredth place tells us to round the 0 up to a 1.
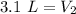
The volume of the neon gas when the pressure is changed to 2.4 atmospheres is 3.1 Liters.