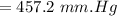Answer:
"457.2 mm.Hg" is the right solution.
Step-by-step explanation:
Given:
Pressure,
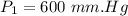
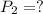
Volume,
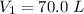
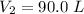
Temperature,

or,
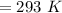
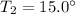
or,
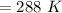
As we know,
⇒
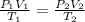
By putting all the given values in the above expression, we get
⇒
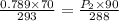
⇒
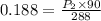
By applying cross-multiplication, we get
⇒
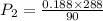
⇒
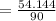
⇒
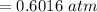
or,
⇒