Answer: The concentration of hydrogen ions for this solution is
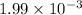 .
.
Step-by-step explanation:
Given: pOH = 11.30
The relation between pH and pOH is as follows.
pH + pOH = 14
pH + 11.30 = 14
pH = 14 - 11.30
= 2.7
Also, pH is the negative logarithm of concentration of hydrogen ions.
![pH = - log [H^(+)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/7qnomb01z9o3qpasap8cj8in33szh8kqym.png)
Substitute the values into above formula as follows.
![pH = -log [H^(+)]\\2.7 = -log [H^(+)]\\conc. of H^(+) = 1.99 * 10^(-3)](https://img.qammunity.org/2022/formulas/chemistry/college/91xlmewwzwvhon963ha3dx4k0jsp07hq42.png)
Thus, we can conclude that the concentration of hydrogen ions for this solution is
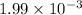 .
.