Answer:
v = 4.233 m/s
Step-by-step explanation:
By applying the rate of boiling from
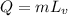 ;
;
the rate of the boiling can be described as:
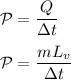
The mode of the steam (water vapor) as an ideal gas can be illustrated by formula:
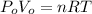 --- (1)
--- (1)
where;
n = number of moles;
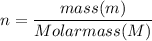
Then; equation (1) can be rewritten as:
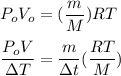
∴
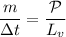
Then:
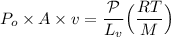
making (v) the subject of the formula:
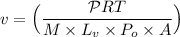
Given that:
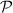 = 0.90 kW = 900 W
= 0.90 kW = 900 W
R(rate constant) = 8.314 J/mol.K
Temperature at 100° C = 373K
For water vapor:
molar mass= 18.015 g/mol ≅ 0.0180 kg/mol
Latent heat of vaporisation
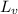 = 2.26 × 10⁶ J/kg
= 2.26 × 10⁶ J/kg
Atmospheric pressure
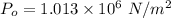
Cross sectional area A =1.60 cm² = 1.60 × 10⁻⁴ m²
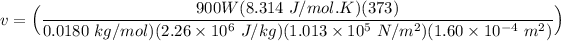
v = 4.233 m/s