Answer:
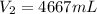
Step-by-step explanation:
Hello there!
In this case, based on the given information, it is possible for us to provide an answer to this question by using the Boyle's law as directly proportional relationship between volume and pressure:
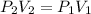
Thus, by solving for the final volume, V2, as required, we obtain the following:
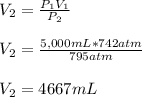
Regards!