You have to go from grams --> moles --> molecules. You can find the equation online.
First you have to find the molar mass of AlF3 which is 83.98 g/mol.
To convert it to moles you simply
450.0 grams ×
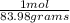 = 5.358 mol
= 5.358 mol
To convert mol to molecules: (multiply by Avogadros # = 6.022*10^23)
5.358 mol ×
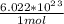 =
=
