Answer:
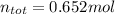
Step-by-step explanation:
Hello there!
In this case, according to the given problem, it is possible to use the given molar masses of NaCl, KCl and LiCl in order to calculate the total moles, yet we calculate the moles of each salt first as shown below:
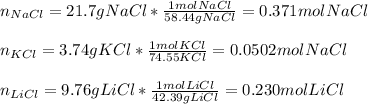
Then, we add them all together to obtain:
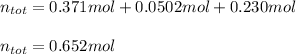
Regards!