Answer:
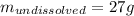
Step-by-step explanation:
Hello there!
In this case, according to the given information of the solubility of copper chloride, as the maximum amount of this salt one can dissolve without having a precipitate, we infer that since just 73 grams are actually dissolved, the following amount will remain solid as a precipitate:
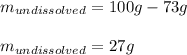
Best regards!