Answer:
The empirical formula of the compound is
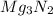 .
.
Step-by-step explanation:
Mass of magnesium in compound = 0.273 g
Mass of nitrogen in the compound = 0.105 g
Moles of magnesium =
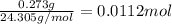
Moles of nitrogen =
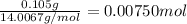
Form empirical formula divides the lowest value of moles of an element present with all the moles of elements present.
Magnesium
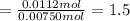
Nitrogen
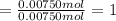
The empirical formula of a compound:
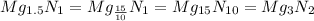
The empirical formula of the compound is
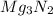 .
.