Answer:
Solution given;
1st temperature [t1]=T
initial temperature [t2]=T+273°C
1st pressure [P]=P
and
initial pressure[P2]=P+20%of P=120/100V=1.2P
now
we have
According to Gay lussac's law
P
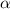 T
T
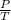 =K where k is constant
=K where k is constant
at 2 different states
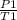 =
=
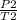
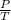 =
=
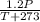
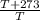 =
=
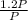
doing crisscrossed multiplication
T+273=1.2T
273=1.2T-T
0.2T=273
T=
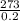 =1365°C or 1365K
=1365°C or 1365K
Initial temperature of the closed vessel is 1365°C or 1365K.