Answer:
Q = 133.76 KJ
Step-by-step explanation:
The amount of heat energy required to raise the temperature of the water can be calculated by the following formula:
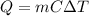
where,
Q = Heat Energy Required = ?
m = mass of water = 2 kg
C = Specific Heat Capacity of Water = 4.18 KJ/kg°C
ΔT = change in temperature = 26°C - 10°C = 16°C
Therefore,
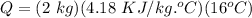
Q = 133.76 KJ