Answer:
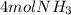
Step-by-step explanation:
Hello there!
In this case, according to the given information it will be firstly necessary to set up the chemical equation taking place:
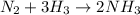
We infer we need to calculate the moles of NH3 by using both of the moles of N2 and H2 at the beginning, in order to identify the limiting reactant:
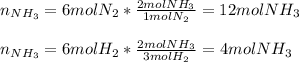
Thus, since hydrogen yields the fewest moles of ammonia, we conclude that we are just able to yield 4 moles of NH3.
Regards!