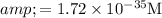Answer:
Step-by-step explanation:
The concentration of copper (II) ions is calculated by expression shown as,
![K_(s p)=\left[\mathrm{Cu}^(2+)\right]\left[\mathrm{s}^(2-)\right]](https://img.qammunity.org/2022/formulas/chemistry/college/947uske7by6kii7kienkm51seltwpgaawv.png)
Here,
The solubility product of CuS is “
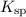 "
"
The concentration of copper ions is "
![\left[\mathrm{Cu}^(2+)\right]](https://img.qammunity.org/2022/formulas/chemistry/college/sd0u13ien4g46a5y8ogoxy1r8eyzefvfcg.png) "$. The concentration of sulfide ions is
"$. The concentration of sulfide ions is
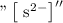
The theoretical value of solubility product of
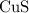 is
is
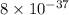
Substitute the known values in equation (I).
![8 * 10^(-37) &=\left[\mathrm{Cu}^(2+)\right] * 0.0464](https://img.qammunity.org/2022/formulas/chemistry/college/pukxjmxikfidh53aywfak7ym5yrjzjk5h4.png)
![\left[\mathrm{Cu}^(2+)\right] &=(8 * 10^(-37))/(0.0464)](https://img.qammunity.org/2022/formulas/chemistry/college/lj10yqjdi62rw9pwlfjjuetkxjnebmdtbo.png)