Solution :
a).
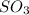
This compound is known as sulfur trioxide.
The molecular shape of sulfur trioxide is trigonal planer.
And the bond angle is 120°.
b).
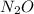
This compound is known as Nitrous oxide. Here, nitrogen is in the center. There is no lone pair around the nitrogen atom and it forms two sigma bonds with the other two atoms.
It is linear in shape.
The bond angle between them is 180°.
c).
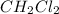
This compound is known as the Dichloromethane.
The molecular shape of the compound is tetrahedral.
The bond angles is 120°