Answer:
a. The resonance effect of the hydroxyl group stabilizes the anionic intermediate
Step-by-step explanation:
The resonance effect stabilizes the the charge through the delocalization of the pi bonds. The resonance stabilization mainly occurs in the conjugated pi systems.
For example, phenol forms a strong hydrogen bonds than the nonaromatic alcohols as the
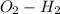 dipole present in the hydroxyl group is being stabilized by the presence of the aromatic ring of phenol.
dipole present in the hydroxyl group is being stabilized by the presence of the aromatic ring of phenol.
Thus the resonance effect of the hydroxyl group stabilizes the anionic intermediate.