Answer:
V = 1.46 L.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to solve this problem based off the definition of molarity, as the moles of solute divided by the volume in liters of solution:
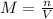
In such a way, we first calculate the moles of K2(MnO4) by using its molar mass of 197.132 g/mol:
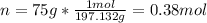
Next, we solve for the volume in the equation of molarity to obtain:
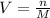
And plug in as follows:
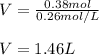
Regards!