Answer:
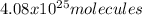
Step-by-step explanation:
Hello there!
In this case, since the understanding of the mole-molecules relationships is analyzed via the Avogadro's number:
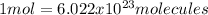
Next, we can solve the following setup by starting with the 67.8 moles of helium and obtaining the required molecules as follows:
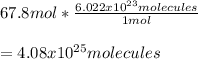
Best regards!