Answer: The average kinetic energy of water at
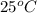 is
is
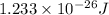 .
.
Step-by-step explanation:
Given: Temperature =
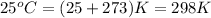
Kinetic energy is the energy acquired by the molecules of a substance due to its motion.
Formula to calculate average kinetic energy is as follows.
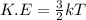
where,
T = temperature
k = Boltzmann constant =
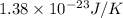
Substitute the value into above formula as follows.
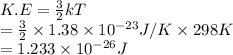
Thus, we can conclude that the average kinetic energy of water at
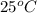 is
is
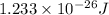 .
.