Answer: The balanced equation is
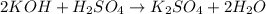 and the concentration of the sulfuric acid solution is 0.184 M.
and the concentration of the sulfuric acid solution is 0.184 M.
Step-by-step explanation:
Given:
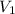 = 27.5 mL,
= 27.5 mL,
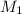 = 0.235 M
= 0.235 M
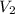 = 35.0 mL,
= 35.0 mL,
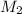 = ?
= ?
Formula used to calculate the concentration of the sulfuric acid solution is as follows.
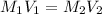
Substitute the values into above formula as follows.
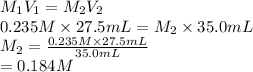
The given chemical equation for given reaction is as follows.

Number of atoms on reactant side are as follows.
- K = 1
- H = 2
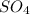 = 1
= 1- O = 1
Number of atoms on product side are as follows.
- K = 2
- H = 2
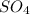 = 1
= 1- O = 1
To balance this equation, multiply KOH by 2 on reactant side and multiply
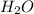 by 2. Hence, the equation can be rewritten as follows.
by 2. Hence, the equation can be rewritten as follows.
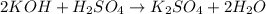
Now, number of atoms on reactant side are as follows.
- K = 2
- H = 4
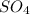 = 1
= 1- O = 2
Number of atoms on product side are as follows.
- K = 2
- H = 4
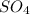 = 1
= 1- O = 2
Since, there are same number of atoms of both reactant and products. Therefore, the equation is now balanced.
Thus, we can conclude that the balanced equation is
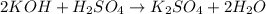 and the concentration of the sulfuric acid solution is 0.184 M.
and the concentration of the sulfuric acid solution is 0.184 M.