Answer:
The chemical symbol will be "
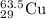 ".
".
Step-by-step explanation:
The given values are:
Current,
I = 2.50 amps
Time,
t = 2 hr
or,
= 7200 sec
Metal deposited,
W = 5.93 gm
As we know,
⇒
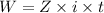
or,
⇒
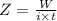
On putting the given values, we get
⇒
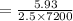
⇒
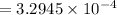
hence,
⇒
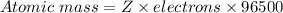
⇒
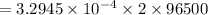
⇒
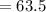
Thus the above is the right answer.