Answer: The pH of resulting solution is 14.
Step-by-step explanation:
pH of a solution is the negative logarithm of concentration of hydrogen ions.
If two or more solutions are mixed together then pH of the resulting solution is calculated as the sum of pH of all the solutions.
As pH of a given solution is 2 and pH of another solution is 12. Therefore, pH of the resulting solution is calculated as follows.
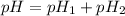
where,
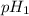 = pH of solution 1
= pH of solution 1
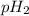 = pH of solution 2
= pH of solution 2
Substitute the values into above formula as follows.
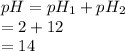
Thus, we can conclude that the pH of resulting solution is 14.