Answer: The event air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down would most likely take place over the next few minutes.
Step-by-step explanation:
A process in which heat is evolved is called an exothermic process.
When hot metal plate at
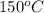 is placed in air at room temperature then heat is given off by the metal plate due to which there will occur a decrease in kinetic energy of its molecules.
is placed in air at room temperature then heat is given off by the metal plate due to which there will occur a decrease in kinetic energy of its molecules.
As a result, molecules in the metal will slow down.
Whereas heat is absorbed by the air molecules from the metal due to which kinetic energy of air molecules will increase.
Thus, we can conclude that the event air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down would most likely take place over the next few minutes.