Answer:
1.06 moles of nitrogen gas will react with 3.20 moles of magnesium in the container.
Step-by-step explanation:
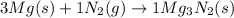
Moles of magnesium heated = 3.20 mol
According to reaction, 3 moles of magnesium reacts with 1 mole of nitrogen gas, then 3.20 moles of magnesium will react with:
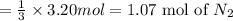
1.06 moles of nitrogen gas will react with 3.20 moles of magnesium in the container.