Answer: There are 0.006 moles of acid in the flask.
Step-by-step explanation:
Given:
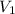 = 21.35 mL,
= 21.35 mL,
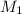 = 0.150 M
= 0.150 M
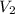 = 25.0 mL,
= 25.0 mL,
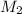 = ?
= ?
Formula used to calculate molarity of
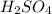 is as follows.
is as follows.
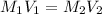
Substitute the values into above formula as follows.
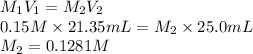
As molarity is the number of moles of a substance present in a liter of solution.
Total volume of solution =
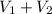
= 21.35 mL + 25.0 mL
= 46.36 mL (1 mL = 0.001 L)
= 0.04636 L
Therefore, moles of acid required are calculated as follows.
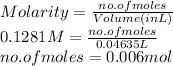
Thus, we can conclude that there are 0.006 moles of acid in the flask.