Answer:
The correct answer is the third option.
Step-by-step explanation:
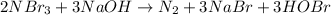
Moles of nitrogen tribromide = 40 mol
Moles of sodium hydroxide = 48 mol
According to reaction, 2 moles of nitrogen tribromide reacts with 3 moles of sodium hydroxide, then 40 mol of nitrogen tribromide will react with:
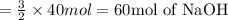
This means that in order to completely react with 40 moles of nitrogen tribromide we will need 60 moles of sodium hydroxide.
But according to the question we only have 48 moles of sodium hydroxide which is less than the 60 moles of sodium hydroxide which indicates that sodium hydroxide is present in a limited amount and nitrogen tribromide is present in an excess amount.
So, the limiting reagent is sodium hydroxide, hence the correct answer is the third option.