Answer: (a) The products are
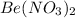 and AgCl. The balanced equation is
and AgCl. The balanced equation is
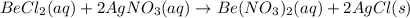 .
.
(b) The type of reaction is double displacement reaction.
Step-by-step explanation:
(a) The complete reaction equation will be as follows.
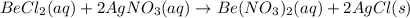
Number of atoms on reactant side are as follows.
- Be = 1
- Cl = 2
- Ag = 2
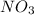 = 2
= 2
Number of atoms present on product side are as follows.
- Be = 1
- Cl = 2
- Ag = 2
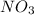 = 2
= 2
Here, number of atoms on both reactant and product side are the same. Hence, it is a balanced chemical equation.
(b) A reaction in which two reactant species tend to change their ions with each other and form new compounds. This type of reaction is called double displacement reaction.
As the given reaction is as follows.
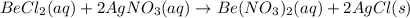
This shows that ions are being exchanges by the reactants
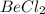 and
and
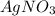 . The ions are
. The ions are
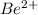 ,
,
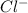 ,
,
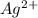 and
and
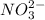 .
.
Therefore, this reaction is a double displacement reaction.