Answer:
613 mg
Step-by-step explanation:
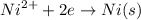
Number of fargday's
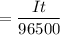
Here, I = 9.20 A
t = 10.5 min
= 10.5 x 60 seconds
So,
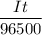
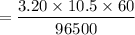
= 0.0208 F
Here, 2e, 2F
2F = 1 mol of Ni
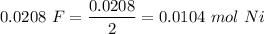
1 mol = 59 gm of Ni
0.0104 mol = 59 x0.0104 gm Ni
= 0.613 gm Ni
= (0.613 x 1000 ) mg of Ni
= 613 mg of Ni