Answer:
The balanced equation for the reaction:
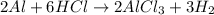
The moles of hydrochloric acid required are 2.61 moles.
Step-by-step explanation:
Aluminum reacts with hydrochlorioc acid to gove aluminum chloride and hydrogen gas as products.
The balanced chemical equation is given as:
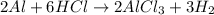
Moles of aluminum = 0.87 mol
According to a chemical reaction, 2 moles of aluminum reacts with 6 moles of hydrochloric acid, then 0.87 moles of aluminum will react with:
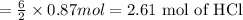
The moles of hydrochloric acid required are 2.61 moles.