Answer:
0.034 M is the molarity of sodium acetate needed.
Step-by-step explanation:
The pH of the buffer solution is calculated by the Henderson-Hasselbalch equation:
![pH = pK_a + \log ([A^-])/([HA])](https://img.qammunity.org/2022/formulas/chemistry/college/oolsmss7kkmfu8ee1rso3ub5gorcgxwxqv.png)
Where:
pK_a= Negative logarithm of the dissociation constant of a weak acid
![[Ac^-]](https://img.qammunity.org/2022/formulas/chemistry/college/x0e6q9tjzygssl93ty49wt3z93tuzl06aa.png) = Concentration of the conjugate base
= Concentration of the conjugate base
[HA] = Concentration of the weak acid
According to the question:
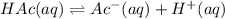
The desired pH of the buffer solution = pH = 5.27
The pKa of acetic acid = 4.74
The molarity of acetic acid solution = [HAc] = 0.01 M
The molarity of acetate ion =
![[Ac^-] = ?](https://img.qammunity.org/2022/formulas/chemistry/college/3cfs9i0s3ixafyuvipg6xjfhpucdmw3mv3.png)
Using Henderson-Hasselbalch equation:
![5.27= 4.74 + \log ([Ac^-])/([0.01 M])](https://img.qammunity.org/2022/formulas/chemistry/college/ofki3uwoitwwaeu1fo0h8xowtqobf9y9un.png)
![[Ac^-]=0.0339 M\approx 0.034M](https://img.qammunity.org/2022/formulas/chemistry/college/7tonn4imtxme1uzb6hxkfxo5r695hippr7.png)
Sodium acetate dissociates into sodium ions and acetate ions when dissolved in water.
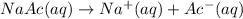
![[Ac^-]=[Na^+]=[NaAc]= 0.034M](https://img.qammunity.org/2022/formulas/chemistry/college/lk1tswhanumdhu18qsbj2ws0l51fjgt6vq.png)
0.034 M is the molarity of sodium acetate needed.