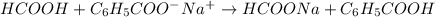Answer: The net ionic equation when formic acid and sodium benzoate are mixed is as follows.
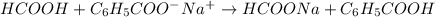
Step-by-step explanation:
A chemical equation in which both reactants and products are present in the form of ions is called an ionic equation.
The chemical formula of formic acid is HCOOH and chemical formula of sodium benzoate is
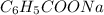 .
.
Therefore, net ionic equation when formic acid and sodium benzoate are mixed is as follows.