Answer: The concentration of KOH is 0.1 M.
Step-by-step explanation:
Given: Mass of KHP = 413 mg (1 mg = 0.001 g) = 0.413 g
Volume of KOH = 20.05 mL
Volume of solution = 41 mL
The molecular mass of KHP is 204.22 g/mol
Hence, moles of KHP are calculated as follows.
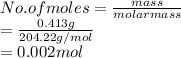
As molarity is the number of moles of a substance present in a liter of solution.
So, molarity of the given solution is as follows.
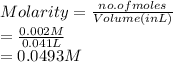
When the given solution is titrated with KOH then concentration of KOH is calculated as follows.
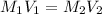
Substitute the values into above formula.
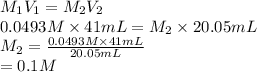
Thus, we can conclude that the concentration of KOH is 0.1 M.