Answer:
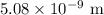
Step-by-step explanation:
h = Planck constant =
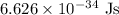
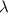 = Wavelength
= Wavelength
m = Mass of electron =
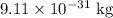
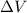 = Potential difference = 14.6 kV
= Potential difference = 14.6 kV
e = Charge of electron =
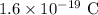
d = Distance between slits
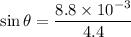
We have the relation
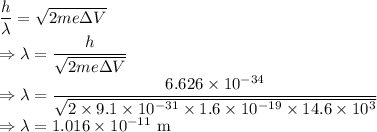
Wavelength is given by
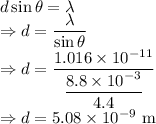
The distance between the slits is
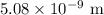 .
.