Answer:
37.1 kilograms of ammonia gas will be produced in this process
Step-by-step explanation:
The percentage yield of the reaction is given by:
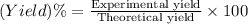
According to question
The percentage yield of the given industrial process = 74.0%
The given theoretical yield of ammonia gas = 50.1 kg
The experimental yield of ammonia gas = x
The percentage yield of the reaction is calculated a:
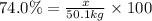
Solving for x, we get:
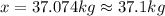
37.1 kilograms of ammonia gas will be produced in this process