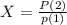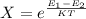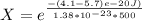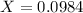Complete Question
A two-state system is in an environment at temperature 500 K. The energy of state 1 is
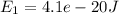 , and the energy of state 2 is
, and the energy of state 2 is
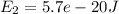 . Let P(1) be the probability of finding the system in state 1, and be the probability of finding the system in state 2. What is the ratio P(2)/P(1)
. Let P(1) be the probability of finding the system in state 1, and be the probability of finding the system in state 2. What is the ratio P(2)/P(1)
Answer:
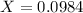
Explanation:
From the question we are told that:
Temperature T=500 K
Energy of state 1
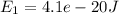 ,
,
Energy of state 2 is
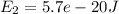
Generally the equation for Probability of finding the system in state is mathematically given by
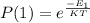
Therefore
For Probability of finding the system in state 1
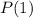
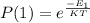
Where
K is Boltzmann constant
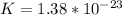
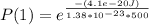
For Probability of finding the system in state 1
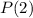
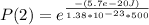
Generally the equation for The Ratio of
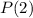 and
and
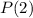 is mathematically given by
is mathematically given by