Answer:
0.535 g
Step-by-step explanation:
The reaction that takes place is:
- NaCl + AgNO₃ → AgCl + NaNO₃
First we calculate how many AgNO₃ moles are there in 25.0 mL of a 0.366 M solution, using the definition of molarity:
- Molarity = moles / liters
- moles = Molarity * liters
Converting 25.0 mL to L ⇒ 25.0 / 1000 = 0.025 L
- moles = 0.366 M * 0.025 L = 0.00915 mol AgNO₃
Then we convert AgNO₃ moles into NaCl moles:
- 0.00915 mol AgNO₃ *
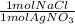 = 0.00915 mol NaCl
= 0.00915 mol NaCl
Finally we convert NaCl moles into grams, using its molar mass:
- 0.00915 mol NaCl * 58.44 g/mol = 0.535 g