Answer:
λ = 11.29 x 10⁻⁷ m = 1129 nm
Step-by-step explanation:
According to the law of conservation of energy:

where,
λ = maximum wavelength = ?
h = Plank's Constant = 6.625 x 10⁻³⁴ J.s
c = speed of light = 3 x 10⁸ m/s
e = charge on electron = 1.6 x 10⁻¹⁹ C
V = stopping poteential = 1.1 V
Therefore,
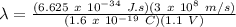
λ = 11.29 x 10⁻⁷ m = 1129 nm