Answer:
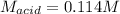
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to firstly write the chemical equation whereby sulfuric acid is titrated with sodium hydroxide:
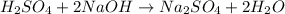
Whereas the mole ratio of acid to base is 1:2 and therefore, the relationship between the volumes and molarities is:
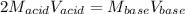
Thus, we solve for the required molar concentration of the acid as shown below:
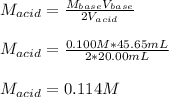
Regards!