Answer:
By measuring out 3.9 grams of cobalt (II) chloride up to a volume of 1.0 L of solution.
Step-by-step explanation:
Hello there!
In this case, considering this question about molarity, it turns out possible for us to calculate the required mass of cobalt (II) chloride by firstly compute the moles present in 1.0L of the given 0.03-M solution:
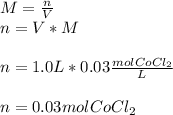
Next, since the molar mass of cobalt (II) chloride is 129.84 g/mol, we compute the grams we need to measure out in order to prepare this solution:
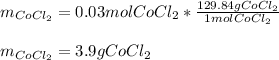
Regards!