Answer:
3) Q = -836.8 J.
4) Q = 950J.
Step-by-step explanation:
Hello there!
In this case, for those calorimetry problems, we use the general equation:
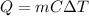
Thus, we proceed as follows:
3) Here, the temperature difference is from 80 °C to 40 °C, the mass is 5.0 g and the specific heat 4.184 in SI units; thus, the heat is calculated as follows:
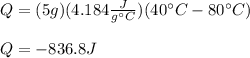
4) Here, the temperature difference is from 100 °C to 200 °C, the mass is 5.0 g and the specific heat about 1.90 in SI units; thus, the heat is calculated as follows:
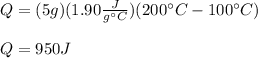
Regards!