Answer: The pressure exerted by the
 gas, in atm is 1.092
gas, in atm is 1.092
Step-by-step explanation:
According to the ideal gas equation:'

P = Pressure of the gas = ?
V= Volume of the gas
T= Temperature of the gas =
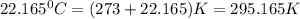 (0°C = 273 K)
(0°C = 273 K)
n= moles of gas =
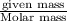
R= Value of gas constant = 0.0821 Latm/K mol
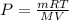 as
as
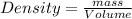
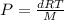 where d is density
where d is density
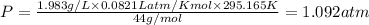
Thus pressure exerted by the
 gas, in atm is 1.092
gas, in atm is 1.092