Answer:
Step-by-step explanation:
Hello there!
In this case, for the given reactants side, we infer this is a double replacement reaction because all the cations and anions are switched around as a result of the chemical change, we infer that the products side include aluminum with nitrate and magnesium with sulfate as shown below:
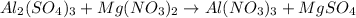
However, we need to balance since unequal number of atoms are present at both sides, thus, we do that as shown below:
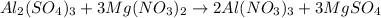
Thus, we make 6 Al atoms, 3 S atoms, 3 Mg atoms and 30 O atoms on each side in agreement with the law of conservation of mass.
Regards!