Answer:
T = 46.97 °C
Step-by-step explanation:
Applying the law of conservation of energy, we get:
Heat Energy Lost by Aluminum Piece = Heat Energy Gained by Water
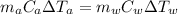
where,
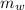 = mass of water = (Density)(Volume) = (1000 kg/m³)(1 L)(0.001 m³/1 L)
= mass of water = (Density)(Volume) = (1000 kg/m³)(1 L)(0.001 m³/1 L)
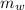 = 1 kg
= 1 kg
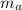 = mass of auminum piece = 2 kg
= mass of auminum piece = 2 kg
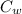 = specific heat capacity of water = 4200 J/kg.°C
= specific heat capacity of water = 4200 J/kg.°C
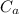 = specific heat capacity of aluminum = 897 J/kg.°C
= specific heat capacity of aluminum = 897 J/kg.°C
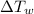 = Change in Temperature of Water = T - 35°C
= Change in Temperature of Water = T - 35°C
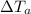 = Change in Temperature of Aluminum Piece = 75°C - T
= Change in Temperature of Aluminum Piece = 75°C - T
T = Final Temperature = ?
Therefore,
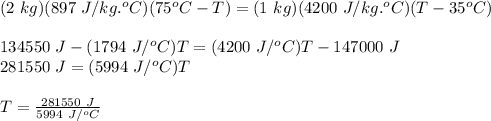
T = 46.97 °C