Answer:
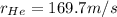
Step-by-step explanation:
Hello there!
In this case, since this problem can be understood via the Graham's law, which states that states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight, which can be extrapolated to the rate, we have:
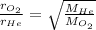
Thus, since the molar mass of helium is 4.0 g/mol and that of oxygen is 16.0 g/mol, we solve for the average velocity of helium as shown below:
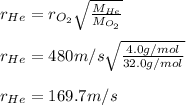
Regards!