Answer:
T= -99 °C.
Step-by-step explanation:
Hello there!
In this case, according to the given description of the problem, it is possible for us to analyze this problem via the ideal gas equation:

However, we first calculate the moles in 3.00 grams of nitrogen gas (28.01 g/mol):
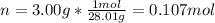
Next, we solve for the temperature as shown below:
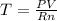
Next, we convert the given pressure and volume to atm and L to obtain:
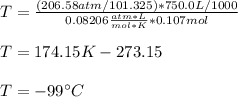
Regards!